A few days and counting: Research and the Future
“Over the past 50 years, people with type 1 diabetes and their medical-care providers have been tantalised with optimism and subsequently disappointed at the seemingly unobtainable cure on the horizon. However, this long journey has been punctuated by several pivotal successes, including the discovery of insulin in 1922, the first pancreatic transplantation in 1966, the first insulin-pump studies, the first immunomodulatory trial in 1986, and the first definitive evidence linking glycaemic control with complication status in 1993. The past 25 years has brought an upsurge of technological advances, including designer insulin analogues, smart insulin pumps, continuous glucose sensors, and closed-loop insulin delivery systems.
Clinicians, investigators, and patients have gained a better appreciation of the true complexity of type 1 diabetes, and humility in the face of many unsuccessful trials aimed at inducing a durable disease remission. While scientists continue to untangle the complicated pathogenesis of the disease, patients and health-care providers should focus on advocating for improved access to modern advances in diabetes care, especially for affordable insulin analogues and technologies that can reduce the burden of managing this chronic disease.”¹
This is a landmark year in which to reach my 50 year diaversary. It coincides with the 100 year anniversary of insulin first being injected into a patient with type 1 diabetes. On 11th January 1922, the first injection of insulin was administered to Leonard Thompson, a 14 year old boy diagnosed with type 1. A second dose was administered on 23rd January 1922. As a result of this trial, type 1 diabetes no longer needed to be considered a terminal illness.
Insulin however is a treatment and not a cure. Injecting insulin leads to many difficulties in managing the condition, as you learn to adapt to a diagnosis of life with type 1.
“Insulin is not a cure for diabetes. It just keeps people alive until we find one.”
American Diabetes Association
So, what does the future hold? Current research falls into a number of categories: disease-modifying therapies, cell therapies or technologies and treatment.
Disease-modifying therapies
Disease-modifying therapies aim to identify those at risk of developing type 1 diabetes and then either reduce the likelihood of or delay the progression to diagnosis.
In 2019, results of the TrialNet² Teplizumab Prevention Study were presented to the American Diabetes Association’s 79th Scientific Sessions. Kevan Herold MD, the study chairman stated “This is the first study to show any drug can delay type 1 diabetes diagnosis a median of 2 years in people at high risk.” In a summary of the results published on their website, JDRF UK explain that:
“Teplizumab interferes with the body’s immune destruction of its own beta cells. While previous studies showed Teplizumab prolonged insulin production in people recently diagnosed, this is the first study to test it in people at high risk for the disease.
Importantly, this study highlights that type 1 diabetes is an autoimmune disease that can be delayed with immune therapy.” ³
Cell Therapy
“Cell therapy aims to introduce new healthy cells into a patient’s body, to replace diseased or missing ones. A challenge for this type of therapy is having enough cells for transplantation into a patient…. Some of these issues can be overcome through the use of stem cells.”
“The ability to turn human stem cells into specialised cell types, such as cells of the brain, eye or pancreas, has opened up possibilities for developing treatments for many degenerative diseases.”
“For cellular therapies to be a clinical success, it is important to have well-established procedures for creating the desired cell types in required quantities. It is also important to ensure that the transplanted cells can survive upon transplantation into the patient and integrate into the body to perform their functions.” ⁴
I was very interested to see a press release in October 2021 entitled “Vertex Announces Positive Day 90 Data for the First Patient in the Phase 1/2 Clinical Trial Dosed With VX-880, a Novel Investigational Stem Cell-Derived Therapy for the Treatment of Type 1 Diabetes.” ⁴ This is very early research and I find that I frequently dismiss headlines like this because the press reporting encourages unrealistic expectations of a cure in the short term. However, this research felt different and exciting. I recognise that the results reported were for one person only, but it was a person and not a mouse! Furthermore, much cell therapy research has focused on newly diagnosed patients whereas the patient in this study had lived with type 1 for around 40 years and prior to the start of the trial, had no measurable insulin production. Yet, by day 90, the patient’s externally administered insulin dose had been reduced by 91% and the patient was managing to achieve better blood glucose targets.
“As a surgeon who has worked in the field of islet cell transplantation for decades, this approach, which obviates the need for an organ donor, could be a game changer,” said James Markmann, M.D., PH,D., Professor of Surgery and Chief of the Division of Transplant Surgery at Massachusetts General Hospital. “We are excited to progress this unique and potentially transformative medicine through clinical trials and to patients.”⁵
There is still a long way to go and the patient in this trial required immunosuppressive therapy. However, several companies are working on research to encapsulate⁶ islet cells. If successful, this would remove the need for immunosuppressive drugs to be taken.
Technologies and Treatments
Technology has been improving my life for the last 40 years. The first big step for me was when I took part in Dr Bill Tamborlane’s early insulin pump research at Yale New Haven Hospital in 1980. The improvements that these early technological devices made in allowing me to test blood glucose levels and deliver insulin in regular doses throughout the day, removed the need for blind adherence to rigid protocols, that did not come close to mimicking the pancreas of a person without diabetes.
Since July 2014, I have combined use of a continuous glucose monitor (CGM) with newer insulin pump technology. The CGM tracks my glucose levels every 5 minutes, sending this data to an app on my mobile phone. This app displays an estimate of my blood glucose value, and the direction and the rate of change.
Over the last 50 years, it is the CGM/pump technology that has had the biggest impact on how well I am able to manage my type 1 - it is life changing. It helps me to make decisions based on how my body has been reacting to life events over the last day or so, and allows me to be more targeted in the treatment decisions that I make. And it works! After time familiarising myself with how best to respond to the data generated, I have been able to reduce my HbA1c and spend more time with blood glucose levels in their target range than ever before. However, for all the advantages there is an overlooked physical and emotional cost.
To obtain the best outcomes in using these devices, I need to be on duty all of the time. No-one else is monitoring the data produced by this equipment. I need to keep a permanent watching brief on my blood glucose data. I have alarms set up on the app on my phone to alert me to when readings are too high or too low. However, I have found from experience that if I wait until the alarms go off, it can be harder and take longer to return readings to a normal range. If I am able to act early, using micro-adjustments to treatment to tackle rising or falling blood glucose levels before I reach readings at the top or bottom end of the normal range, it is easier to avoid readings outside the normal range. But this non-stop monitoring of data, without a break, is exhausting.
Research over the last five years has been looking at how we can improve the use of blood glucose data and relieve the burden of managing the condition by using closed-loop insulin delivery systems.
“Closed-loop insulin delivery systems (also known as ‘artificial pancreas’ systems) take the technology to the next level by integrating continuous glucose monitoring with an insulin pump and an algorithm which automates insulin delivery. Hybrid closed-loop systems are characterised by the coexistence of automated insulin delivery (via the algorithm) and user-initiated insulin delivery, for example, providing mealtime boluses.” ⁷
In 2017, a clinical trial by Dr. Roman Hovorka, University of Cambridge Metabolic Research Laboratories, Wellcome Trust-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge, UK, and colleagues concluded that patients using the hybrid closed-loop therapy were shown to have a higher percentage of time with blood glucose levels within their target range and a reduced HbA1c.
The authors conclude: “The use of day-and-night hybrid closed-loop insulin delivery improves glycaemic control while reducing the risk of hypoglycaemia in adults, adolescents and children with type 1 diabetes compared to conventional pump therapy or sensor-augmented pump therapy. Results from our study together with those from previous studies support the adoption of closed-loop technology in clinical practice across all age groups.”
“Dr Hovorka’s study is significant in that it adds to the ever-growing body of evidence showing that closed-loop insulin delivery systems improve outcomes and reduce burden for people with type 1 diabetes,” said Daniel Finan, Research Director at JDRF, who supported the study. ⁷
My Future
I have referenced only a handful of projects above but articles in the medical press show that a great deal of exciting research is in progress or in the early stages of seeking funding. Unfortunately, shortages of available funds also mean that many good projects are unable to get support.
My short-term hopes for the future centre around gaining access to a hybrid closed-loop (HCL) system. My current pump is out of warranty in July 2022 and I would like an HCL system to help manage the burden of constantly aiming for in-range blood glucose values. The most appealing benefit of these systems for me is the help they give in managing blood glucose levels overnight. No matter how long I have lived with type 1 and how well health care professionals feel I manage, I can still have problems with overnight blood glucose levels, regardless of how careful I am in adhering to the tried and tested patterns of behaviour to try to minimise the risks of problems.
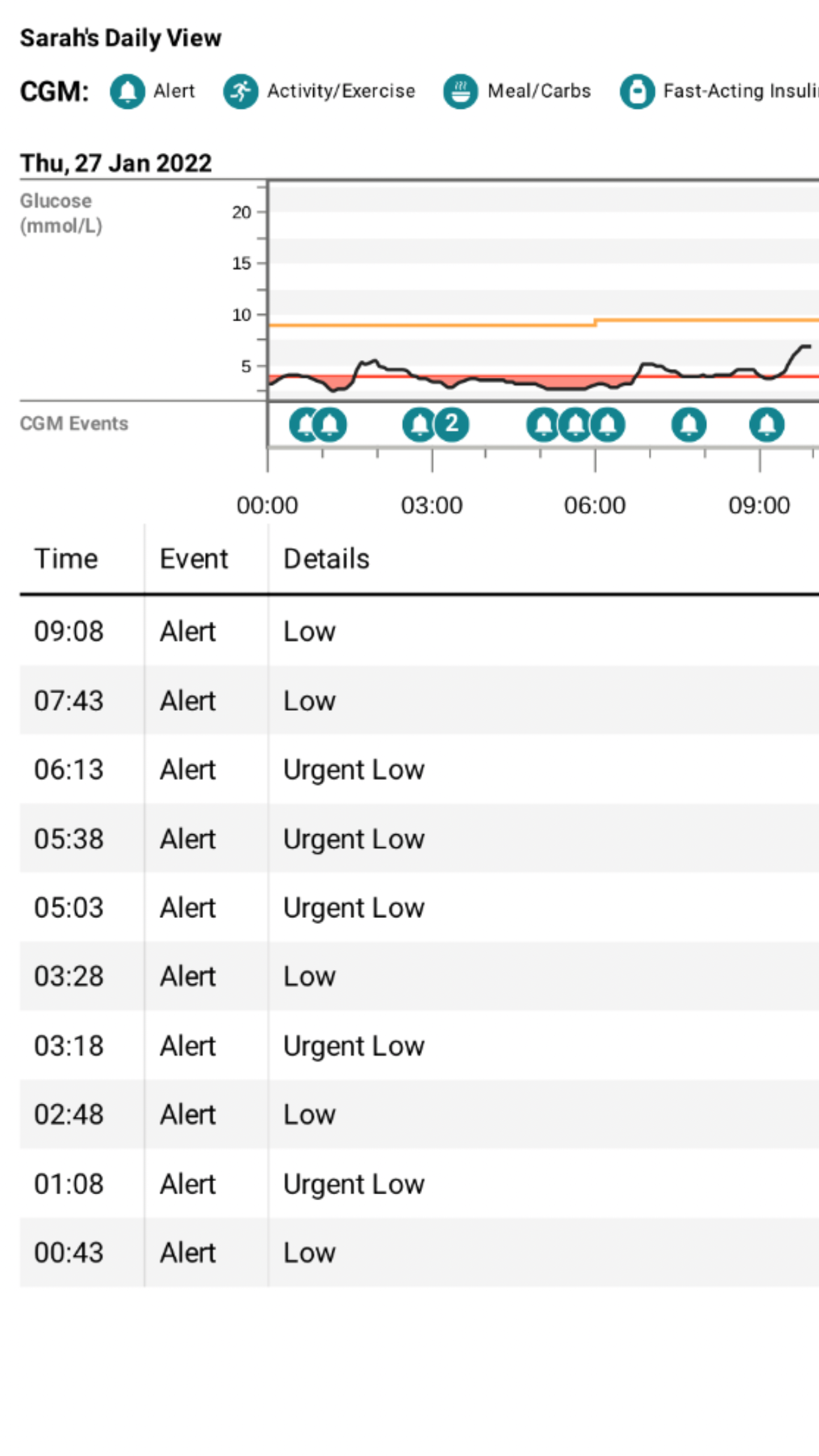
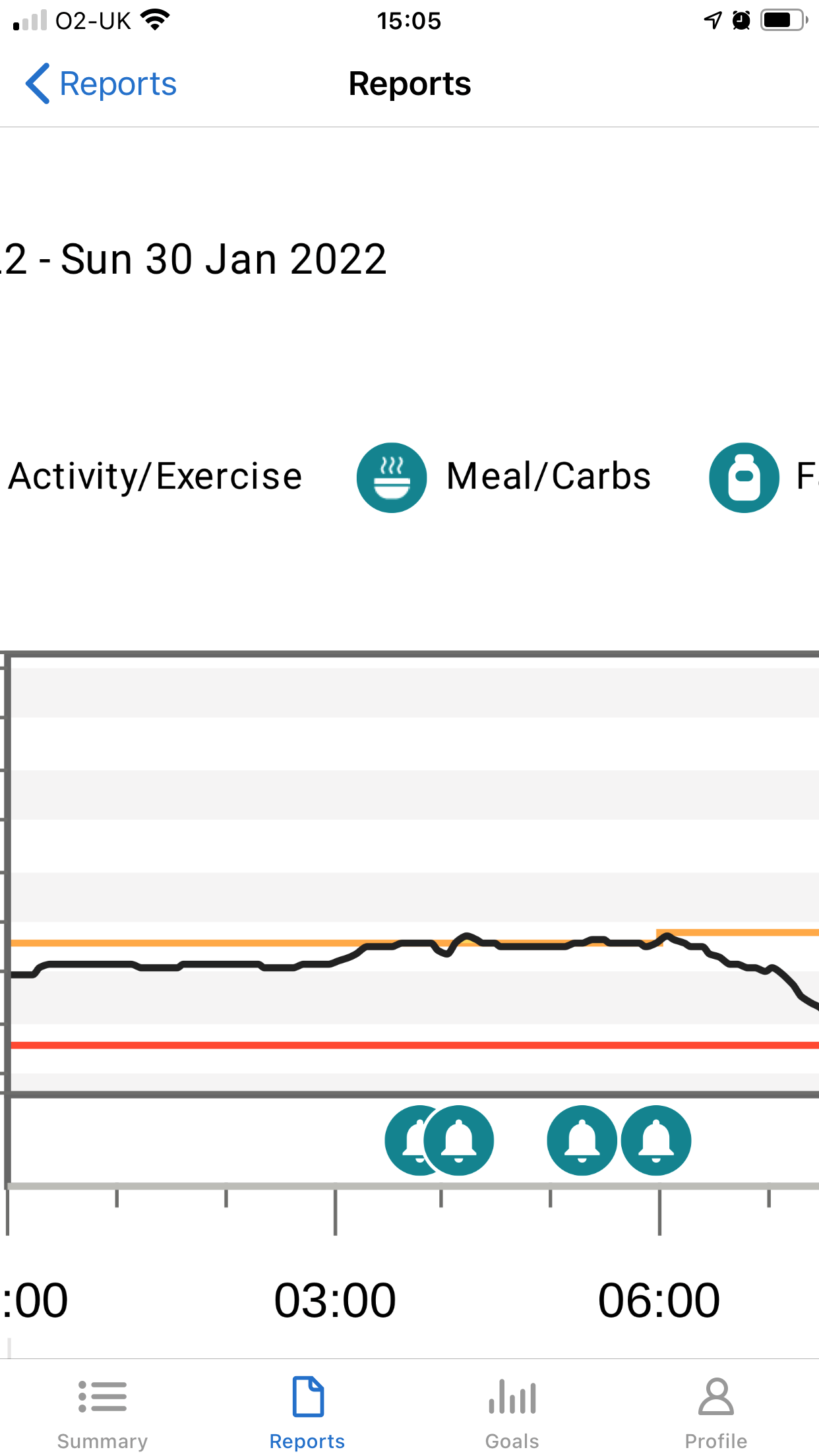
My recent experiences on two consecutive nights at the end of January can perhaps help to explain some of the issues that may go unseen. I don’t know why I had two such bad nights. The previous day had been the same as usual, my food intake had been the same as usual, there was no more work stress than usual and my activity levels were the same as usual. However, between midnight and 6am on the 27th January, I was woken by 8 alarms from my CGM. Despite my consumption of multiple jelly babies and orange juice throughout the night, my blood glucose levels remained low. Getting through the next day at work required all the grit and determination I could muster. Determined not to have another night like that, I reduced the background insulin dose on my pump by 0.05u per hour during the following night and the opposite problem occurred. I had 4 alarms from my CGM between 3am and 6am telling me that my blood glucose levels were in double figures and too high.
I spent the weekend recovering – including more time in bed to recover my lost sleep and to ensure that I had sufficient energy levels to get through the next week. I have a long list of things that I would like to do away from work but doing them can be pushed back from week to week, until I feel that I have the energy to devote to this side of my life. When the technology already exists to help limit the impact of overnight blood glucose variability on my lifestyle, it is frustrating that access is made so difficult.
When I speak to health care professionals, they conclude that I am managing well. So many treatment decisions are based on my HbA1c result. I am able to achieve an HbA1c below 6.5%, so I am deemed to be managing well. My percentage of blood glucose time in range supports this view. However, none of this takes into consideration overnight episodes like the one I described above. That episode will not be classed as severe hypoglycaemia as I dealt with the constant low alarms myself. Yet, the impact of episodes like that on my ability to function at work, to cope with the disruption to my sleep and to have the energy to want to participate in activities away from work is significant for my quality of life.
Much of the official guidance surrounding access to technology currently states that its provision should be “considered” as opposed to “offered”: because funding is in short supply, considering my request for access to technology has frequently resulted in the answer “no”.
Funding is currently being sought for exciting research at Exeter University. I was recently asked if I would undertake a PPI (Patient and Public Involvement) review of a grant application being made by Sarah Richardson, an Associate Professor in Cellular Biomedicine at the University of Exeter Medical School. This line of work considers how therapies could be better targeted, recognising that T1D may not be just ‘one disease’. She stated that,
“Using rare type 1 diabetes (T1D) pancreas biobanks, we have previously shown that T1D is not the same in every individual. We have demonstrated that at disease onset, the number and type of immune cells observed in and around islets that still contain beta cells, differs between very young children and those diagnosed in their teens. It is also evident that, in the older group, many more beta cells are present than predicted, suggesting that some may be dysfunctional rather than destroyed. Crucially this suggests there are differences in the mechanisms causing diabetes and that therapies for T1D must be targeted to the specific form of diabetes an individual has.
To better understand these differences these precious and rare samples from biobanks across the world need to be carefully analysed in the same way. These harmonised analyses must be made easily accessible to researchers in the field of T1D who are not necessarily familiar with T1D pathology. This would deepen understanding and appreciation that there are different disease forms and encourage the field to consider more patient-specific targeted therapies, to tailor the management of the condition to the individual, and to stop thinking of T1D as being ‘one disease’.
For those who live with diabetes currently, this research has the potential to inform their clinical management and alter future treatment options within the next 2 to 4 years. For example, individuals diagnosed with T1D later in life could benefit from treatments aimed at re-awakening or reviving the functional capacity of their remaining beta cells. In contrast, those diagnosed at younger ages could be directed towards current, ongoing immunotherapy trials which aim to target specific immune cell subtypes or immune cell interactions soon after clinical onset.
In those individuals diagnosed very young but with a longer duration of diabetes, these findings could provide valuable supporting evidence for the need to improve access to diabetes technology (pumps, CGM) to help individuals manage their condition. For those at-risk of developing type 1 diabetes in the future, this research is likely to provide critical information related to pancreas dysfunction in the pre-diabetes period and at onset. This information will be key to helping researchers to identify appropriate targeted immunotherapies, tailored to the underlying disease processes.”
I continue to remain optimistic and am hopeful that support for both JDRF and Diabetes UK will enable advances in research that will continue to improve both the quality of life and the outcome for everyone living with type 1 diabetes. Much as I would love a cure, given that one has yet to be found, I am thrilled to be reaching my 50 year diaversary. It is difficult to know how many people I will share this achievement with. The most recent data that I was able to obtain from the National Diabetes Audit 2019-20⁸, published in August 2021, included statistics for 218,670 people in England and Wales confirmed as having a diagnosis of type 1 diabetes. The report stated that results should be taken in the context of low data submission from specialist services, probably hampered by covid-19, but for these numbers reported, 17,650 (8%) had been diagnosed for between 40-49 years and 9,085 (4%) had been diagnosed for 50 years or more.
A number of health care professionals have told me in conversations that it is becoming more common to see patients diagnosed with the condition for more than 50 years. That is a real positive. It offers me hope and reinforces how the improvements in treatment over recent years are contributing to improving life spans for those of us living with the diagnosis.
The build up to my 50 year diaversary has encouraged me to take stock and I have come to realise that I have much to feel proud of in how I have managed my life with the condition. I will continue to look ahead. Diabetes UK produce medals recognising 60, 70 and even 80 years of life with type 1 diabetes. I look forward to reaching those milestones too.
Notes
1. Type 1 Diabetes; Linda A DiMeglio, Carmella Evans-Molina, Richard A Oram
US National Library of Medicine, July 2019
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6661119/
2. Type 1 Diabetes TrialNet; website viewed January 2022
https://www.trialnet.org/our-research/completed-studies/teplizumab
General information about TrialNet and the screening programmes that they offer can be found on their website
3. JDRF UK website; website viewed January 2022
“Immunotherapy delays type 1 diabetes diagnosis in people at high risk”
Published 9 June 2019
https://jdrf.org.uk/news/immunotherapy-delays-type-1-diabetes-diagnosis-in-people-at-high-risk/
4. British Society for Gene and Cell Therapy; website viewed January 2022
“What is Cell Therapy?”
https://www.bsgct.org/education/what-is-cell-therapy.aspx
5. Vertex Pharmaceuticals Incorporated website; viewed January 2022
6. JDRF UK website; viewed January 2022
“Encapsulation”
https://jdrf.org.uk/our-research/about-our-research/cure-research/encapsulation/
7. JDRF website; Press release 3-Oct-2018; viewed January 2022
“Closed loop ‘artificial pancreas’ insulin delivery system offers better glucose control and reduced risk of hypoglycemia”
8. National Diabetes Audit, 2019-20, Type 1 Diabetes
NHS Digital, England and Wales; published 12th August 2021