36 weeks and counting: Monitoring
Me: In 1972, when I was discharged from hospital, the only way to monitor how well my insulin dose was matching my needs as a type 1 diabetic was by performing a urine test. We had a wide plastic measuring jug that sat permanently on the cistern of the downstairs toilet. I did a fasting urine test every morning, when I would put 5 drops of urine, followed by 10 drops of water, into a glass test tube. I added a Clinitest tablet to this mixture and it would effervesce before settling to display a colour. I had to match the colour to a chart, and I wrote the reading into the relevant column of a notebook that Mum had carefully ruled. She created record diaries for me to complete in small hardback notebooks, with columns to record urine test results, the morning insulin dose given and any other notes to help determine possible causes for issues that may have arisen. I had coloured felt tip pens to use to record my readings. Although not great from a management point of view, I had plenty of blue, green, brown and orange entries throughout those early years. I think, even then, I knew that there were “bad” colours and would almost hold my breath while waiting for the result, willing it not to be brown or orange.
It could sometimes be difficult to wee on demand and so Mum would turn on the tap in the bathroom hoping that the sound of running water would help.
Dad: We made regular measurements of the sugar in her urine and kept charts of the readings. Rough and inaccurate as it was, it was the accepted method in those days of monitoring progress. To our relief, Sarah appeared to remain in good health.
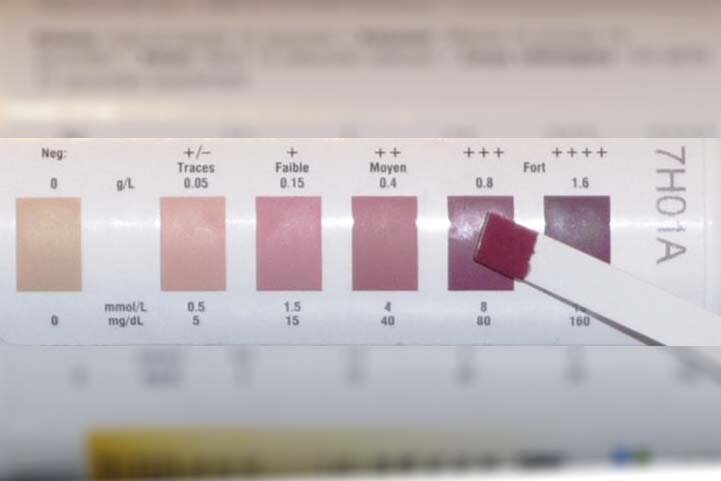
Me: I was later provided with reagent test strips to dip into urine to measure either glucose or ketone¹ levels. The testing strips gave results more quickly than Clinitest tablets. I would dip the sticks into my urine, count a set number of seconds and then compare the strip to the colour chart on the side of the jar. The ketone test strips returned varying shades of purple if ketones were present and I would record the presence of ketones with +, ++ or +++ in the record book next to the colour denoting the level of glucose in my urine.
Mum: We aimed for the magic “blue” sugar-free colour for your urine tests by following all the “rules”. However, results were variable from one day to another. Looking back, I think much of your early care, whilst very good, was somewhat “hit and miss”. We had regular hospital clinic visits and an annual short-term stay, but no contact in between appointments. Any changes made to your regime at one hospital appointment had to wait until the next visit to be assessed, and then tweaked if appropriate. There was no one to turn to for instant advice.
Me: The problem with urine testing, as a method for monitoring type 1, is that it gives only an indirect indication of what you need to know. The test reflects what was likely to have been happening several hours previously. The other key issue is that glucose tends to appear in the urine only when blood glucose levels are over 10 mmol/l. Given that general guidance today is for type 1’s to aim to maintain blood glucose levels in the range 4-7 mmol/l pre meal and 8-9 mmol/l range up to two hours post meal, a urine test is insufficiently sensitive and current for managing the condition.
In preparation for outpatient clinic appointments, I had to undertake 24 hour urine collections. On these days I would carry two large plastic storage containers with screw top lids on the bus into school. The storage containers would be kept in the school office and I would need to collect them before every visit to the toilet, pouring what I collected into one of the storage containers, before returning the containers to the school office.
Blood testing was performed only at regular outpatient clinic appointments, where my urine testing record would also be examined, specific concerns discussed and insulin doses adjusted by the doctor. The very basic injection regimes meant that dose adjustments were not made at home, because any adjustments were incapable of correcting your body’s response to situations quickly enough.
Mum: After your care was transferred to the paediatric diabetic team at Pendlebury hospital, in addition to outpatient clinic appointments, the monitoring undertaken included an annual inpatient stay for a minimum 24 hour period, to enable more detailed observations of blood glucose levels. This also gave us an opportunity to try to understand what was causing your episodes of night-time hypos and fitting. (More of this in a later blog).
Me: Inpatient stays were fitted into school holidays so I invariably went into hospital in August. Every August, new medical graduates moved into a range of posts in hospitals to, as the NHS website states, practise and gain competence in basic clinical skills. On one inpatient stay, I remember being woken by a young doctor tasked with obtaining a blood sample. He struggled to find a vein, probably not helped by a child unhappy at being woken and had to call on assistance from another doctor. By this stage, the doctors’ attempts had left me crying loudly. They admitted defeat and allowed me to return to sleep. Remembering the episode, and how vocal I was, often makes me wonder whether the young doctor found the episode equally traumatic.
Having moved to the US with my family in October 1978, I was invited to take part in early insulin pump research conducted by Dr Bill Tamborlane at Yale University Medical School. The research required continuing with urine tests before each meal and at bedtime. However, I was now asked also to undertake home blood glucose tests to be performed between four and six times a day on three days each week. For the purposes of the research, I was issued with my first blood testing meter. I remember its having pride of place in the corner of the counter top in the downstairs bathroom. I was grateful that there was a counter top surrounding the washbasin and more space than in our downstairs toilet in the UK, as, in addition to the meter, I would sometimes be asked to collect blood samples prior to hospital visits. I would have a tray of yellow lidded small plastic tubes to fill at varying times of the day, alongside my meter, making the downstairs bathroom look more like a mini laboratory.
So, finger pricking became the new challenge. There were no automatic finger pricking devices as there are today, that allow you to obtain a blood sample by setting the depth at which the lancet pricks your finger at the push of a button. Instead, I had individually wrapped foil packets, each containing a lancet with a rectangular metal base to hold, topped with a triangular blade. I had to pluck up courage to stab myself and using a triangular blade, rather than the thin needles used today, meant that if you stabbed yourself too deeply, you would make quite a wide cut. Trying to gauge just how hard to stab yourself took quite some practice, especially because it is a natural human instinct to protect yourself from pain. If you approached the task with hesitancy, the cut would not allow enough blood to be obtained and too deep a stab was painful and messy, with a resulting finger “gusher”.
Despite these challenges, blood testing was an improvement in managing the condition. Machines were not portable and would not accompany me out of the home, but, when used to test, could give you a current reading that could help to explain how you felt and start to allow better decision making around appropriate insulin doses. Whereas a blue urine test might mean that you had a blood sugar reading anywhere below 10mmol/l up to two hours ago, a blood test could give you a reading of your blood glucose now, and could differentiate between a reading verging on too low (below 4 mmol/l), in range (in the 5-7 mmol/l) or higher than desirable (9-10 mmol/l).
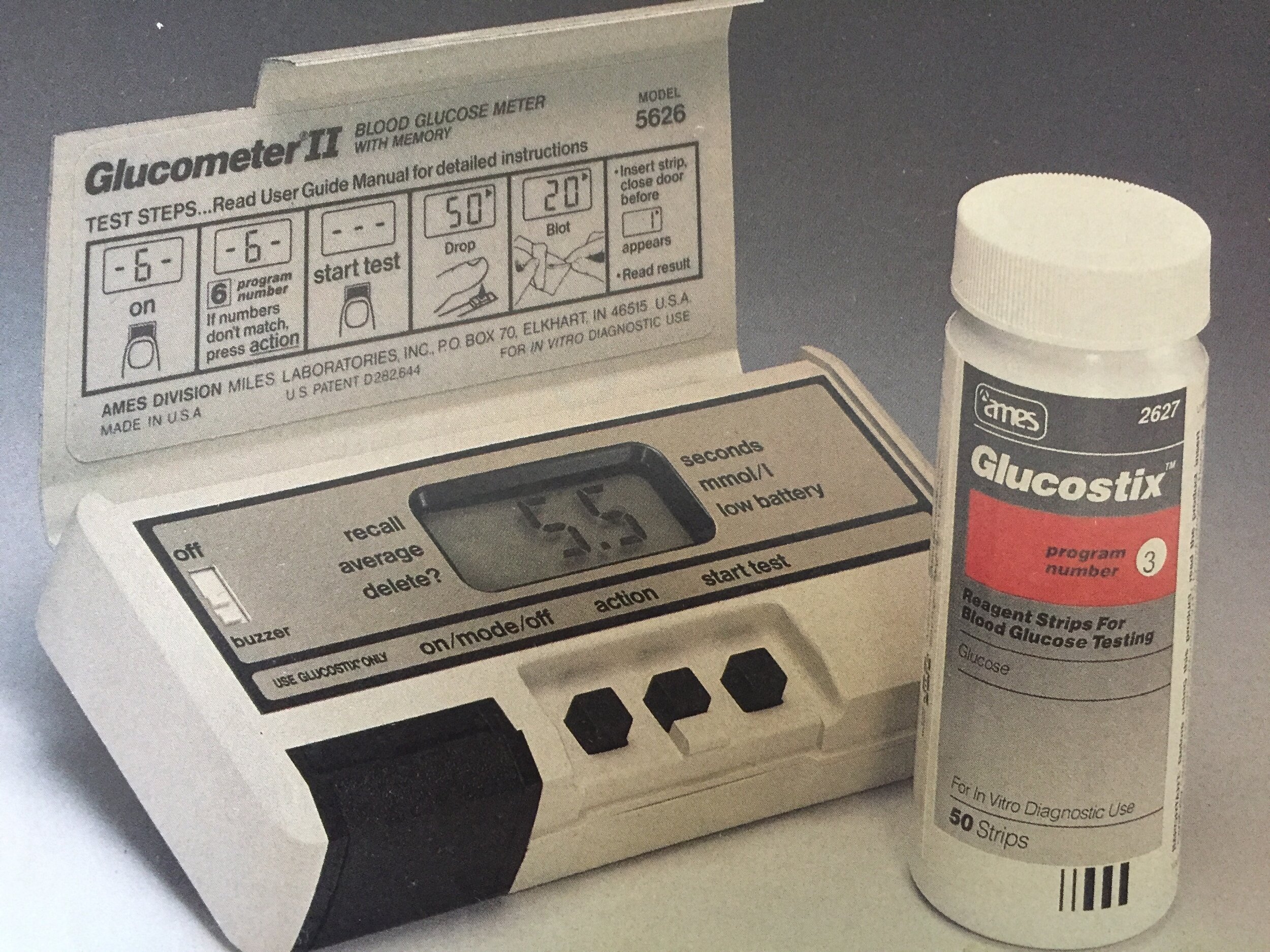
On returning to the UK in 1981, we obtained a Glucometer. It was another colossal blood testing machine that looked and felt like a brick. A blood test would take around a minute to perform, but was nevertheless preferable to urine testing. The bulkiness of the kit meant that it didn’t accompany me to school and similarly, when I headed off to university, it stayed in my accommodation. Blood testing machines started to improve rapidly throughout the late 1980’s and early 1990’s offering machines that were portable and tests that would often take only 5 seconds and require a smaller sample of blood. Finger pricking devices went through similar transformations, becoming automatic devices that could be adjusted for depth of penetration and would operate at the push of a button.
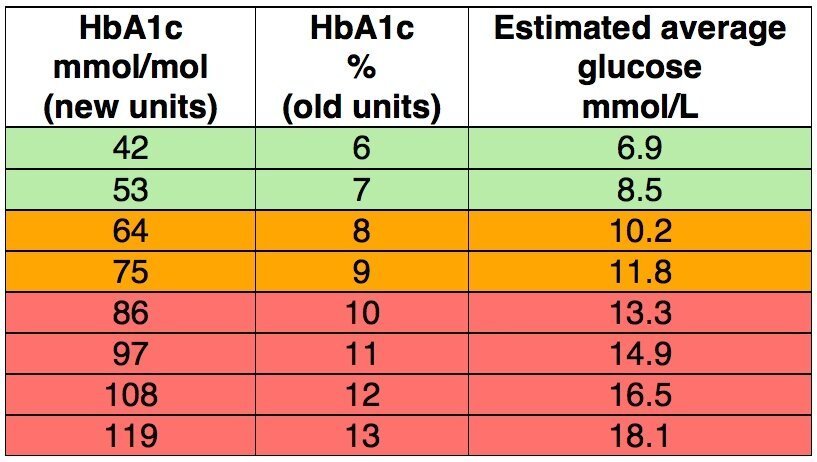
Hospital monitoring was also seeing changes. HbA1c blood tests, giving you an overall picture of your average blood glucose levels for the last two to three months, were being introduced into clinical settings. Out of curiosity I emailed Dr Tamborlane several years ago to ask if my records from the insulin pump research would still be available. Although the records had not been kept, he remembered that most children taking part had average HbA1c levels of around 9.5% at the start of the trial and following pump usage, these readings had reduced to around 7.5%.
The value of more intensive management, involving 3 or 4 injections a day or pump usage, and home blood glucose testing, compared to 1 or 2 injections a day and urine testing, was investigated during the 1980’s and early 1990’s by the Diabetes Control and Complications Trial (DCCT). The trial ended in 1993, a year earlier than planned when the study showed that where participants were able to keep their blood glucose readings as close to normal as possible with these improved tools, their risks of developing the long-term complications associated with type 1 diabetes were significantly reduced. It is generally accepted that an HbA1c result below 6% equates to that of a non-diabetic.
This was very much the way monitoring stayed for me for some time: home blood glucose testing, HbA1c screening at six monthly appointments and annual eye screening to monitor for changes that might show early signs of diabetic retinopathy. In my little bubble, receiving annual GP check-ups away from hospital outpatient clinics, I was unaware of the monitoring transformation taking place.
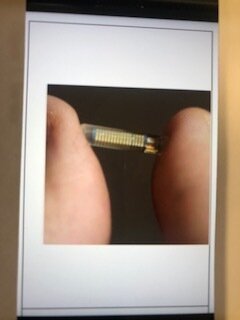
I managed to get myself fitted with an insulin pump in 2010, and, reading its instruction manual, I found a section on continuous glucose monitors (CGMs). Having set up the pump, I was equally keen to read up on the Enlite CGM made by Medtronic. CGMs work through a small 9mm sensor wire that is inserted into the skin and takes glucose readings from interstitial fluid every few minutes. A wireless transmitter attached to the sensor sent the readings to the pump. Although the pump could not use the blood glucose readings to adjust the insulin doses, the system nevertheless provided a 24 hour graph identifying trends in your estimated blood glucose levels. So, in theory, it could enable you to better match your insulin dosing to your body’s needs. This sounded life changing. Unable to qualify for an NHS funded CGM, I contacted Medtronic, bought a CGM starter kit and set about teaching myself how to use it.
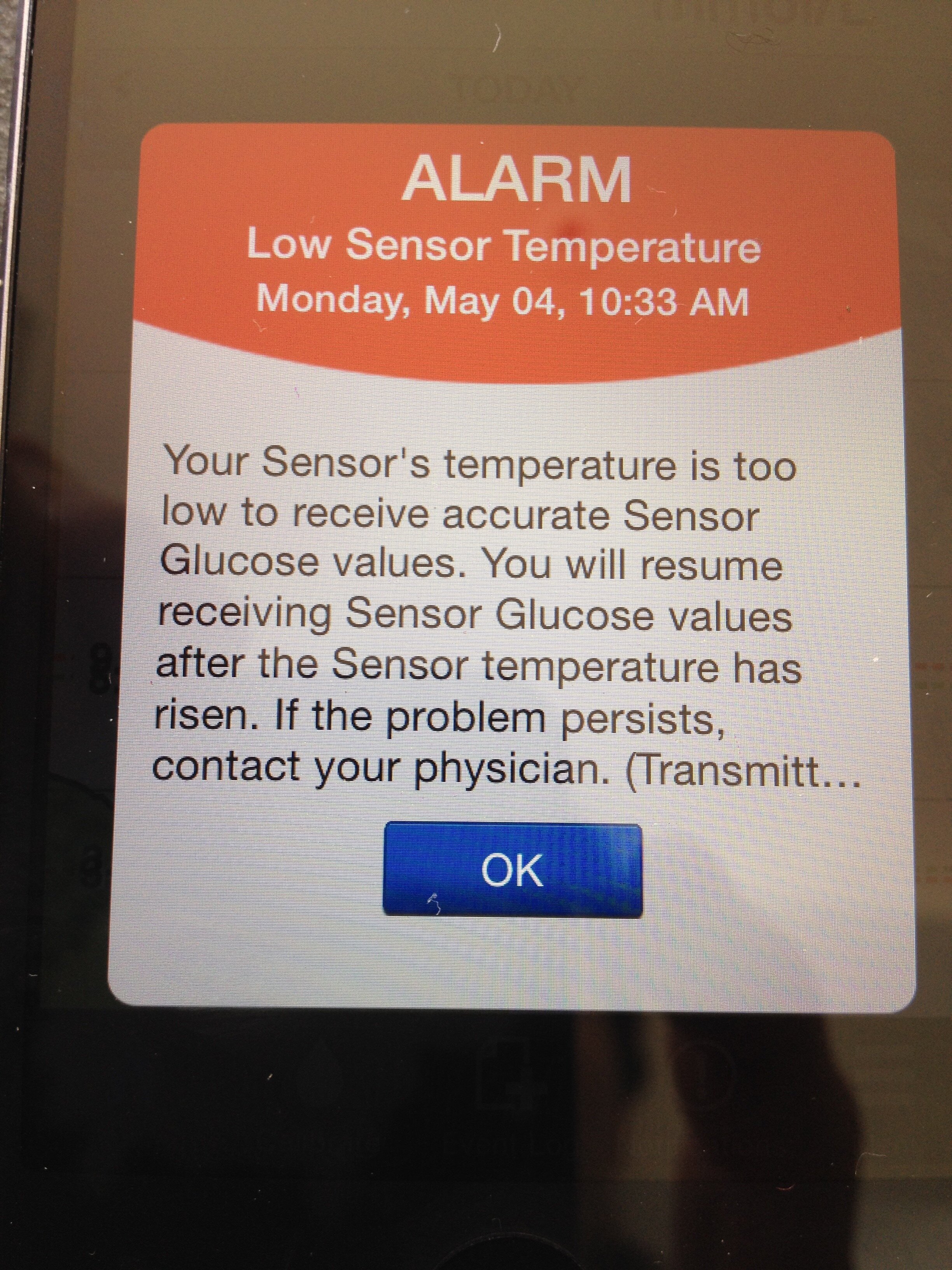
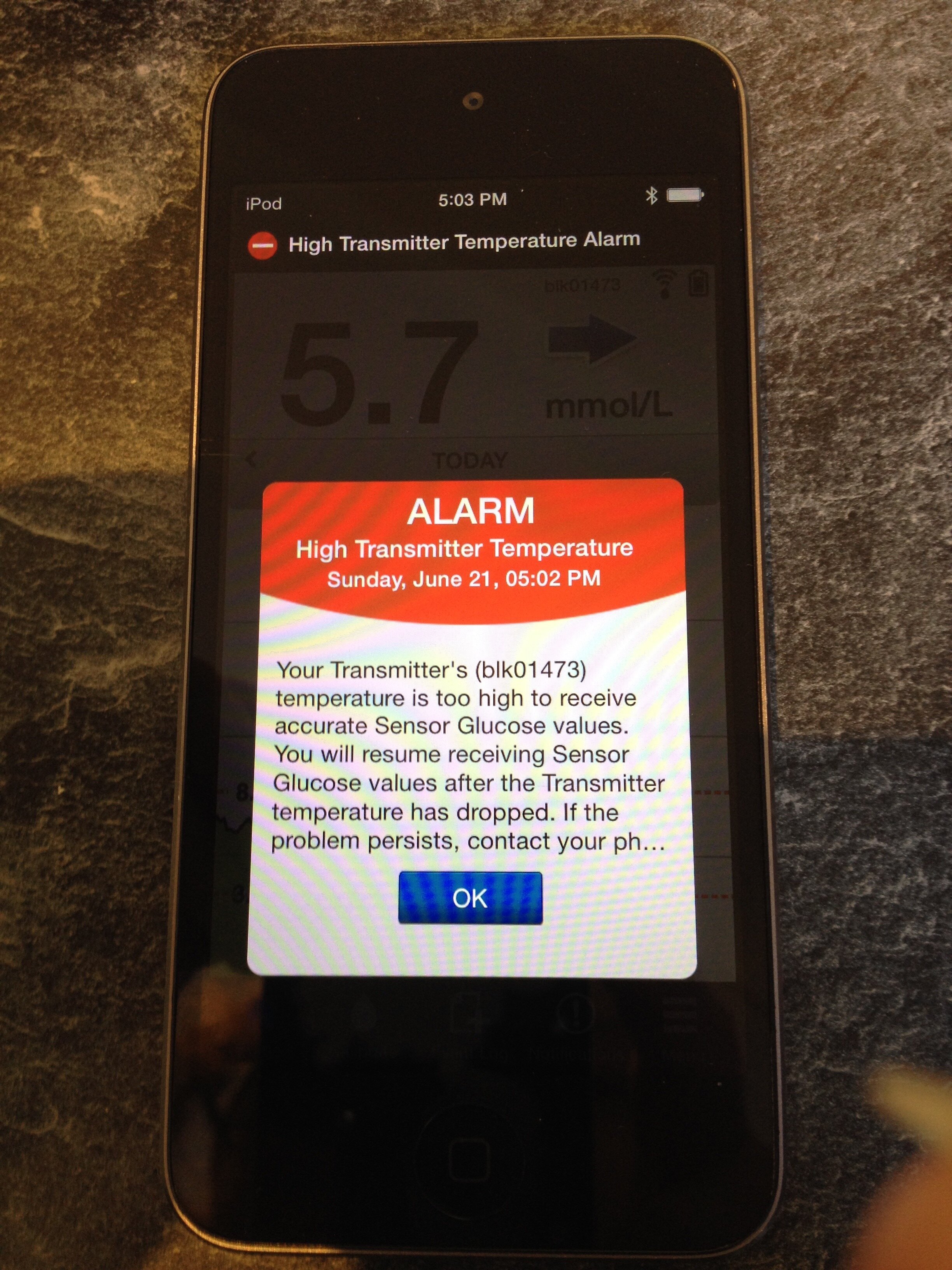
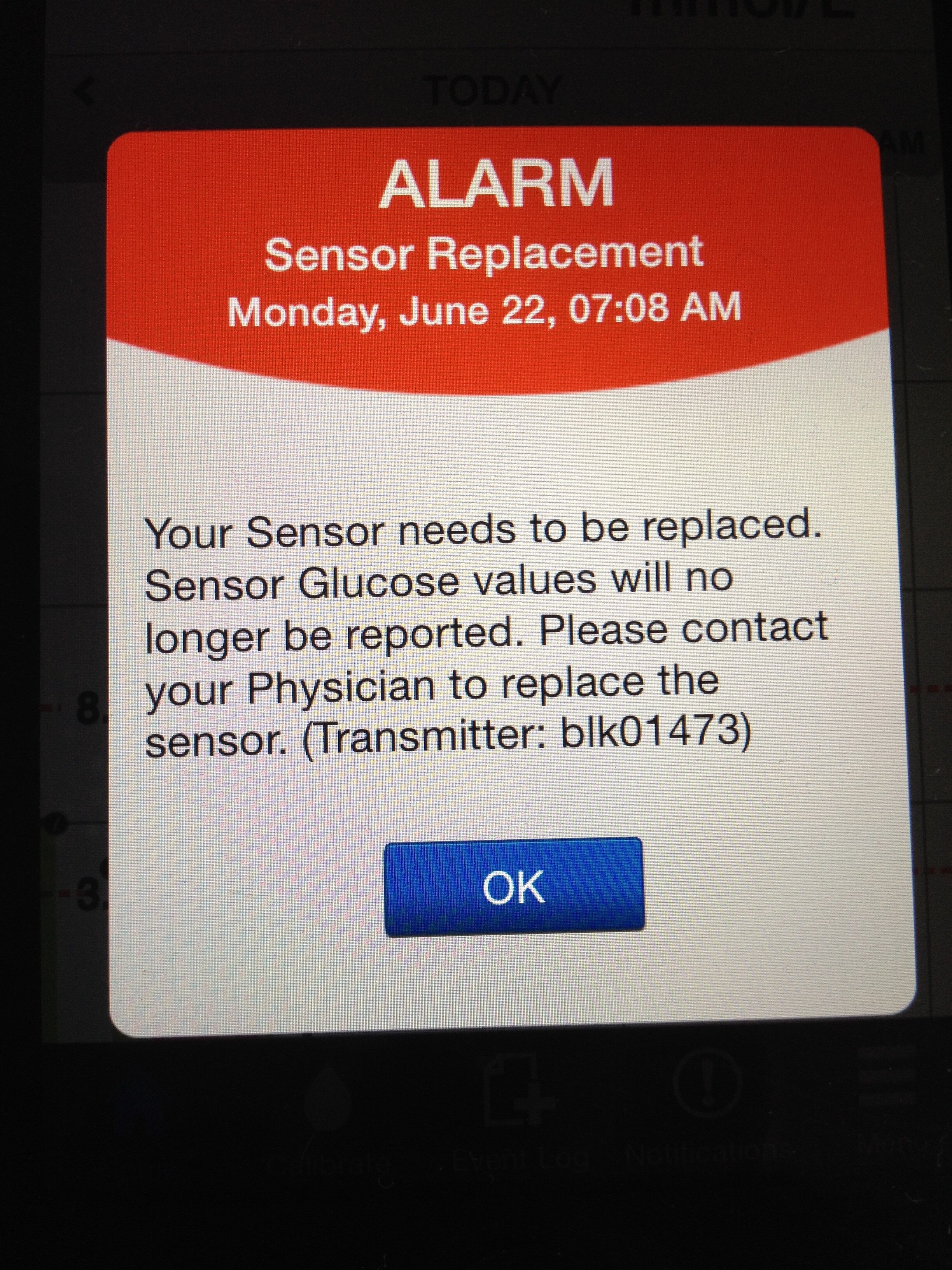
Unfortunately, this early CGM usage did not fully live up to expectations. Sensors lasted only 6 days before a replacement was needed (quite a financial drain) and they had to be calibrated twice a day with a home blood glucose test. Their readings were not approved as an alternative to blood glucose readings and were not to be used for making dosing decisions. However, the historic trend lines could be a revelation in providing information to help manage the condition. They would help me see more clearly how blood glucose was affected by daily life and would give me an idea of what happens overnight. The system included an alarm function and could alert you to out of range blood glucose readings. Despite calibrating the sensors twice daily and understanding good calibration technique, I did not find them to provide an accurate approximation to blood glucose trends. I would find results from the sensors to be too different from the blood test results for me to be confident in using them for decision making and alarms would sound incorrectly alerting me to problems that were not there. After persevering for a year, I gave up and returned to blood glucose monitoring only.
A second look at the possibilities that CGM could provide came in 2014. My husband Neil ran the London Marathon for the Juvenile Diabetes Research Foundation (JDRF). When signing in at their registration desk, he struck up a conversation with a volunteer who enthused about Dexcom CGM sensors. I had booked myself onto a JDRF Discovery Day the following month to hear Roman Hovorka talk about the research being funded by JDRF into closed loop systems. When I arrived, I asked the organisers if they knew any attendees using this Dexcom CGM. The rest is history! I was pointed in the direction of three different people, active in the type 1 community, who were happy to talk to me about their experiences. (More on the wonderful type 1 community in a future blog).
Although I would still not receive NHS funding, I decided to try again and in July 2014, took my first delivery of Dexcom G4 Platinum sensors. Over the last 7 years I have experimented with a variety of CGM type devices all with different pros and cons:
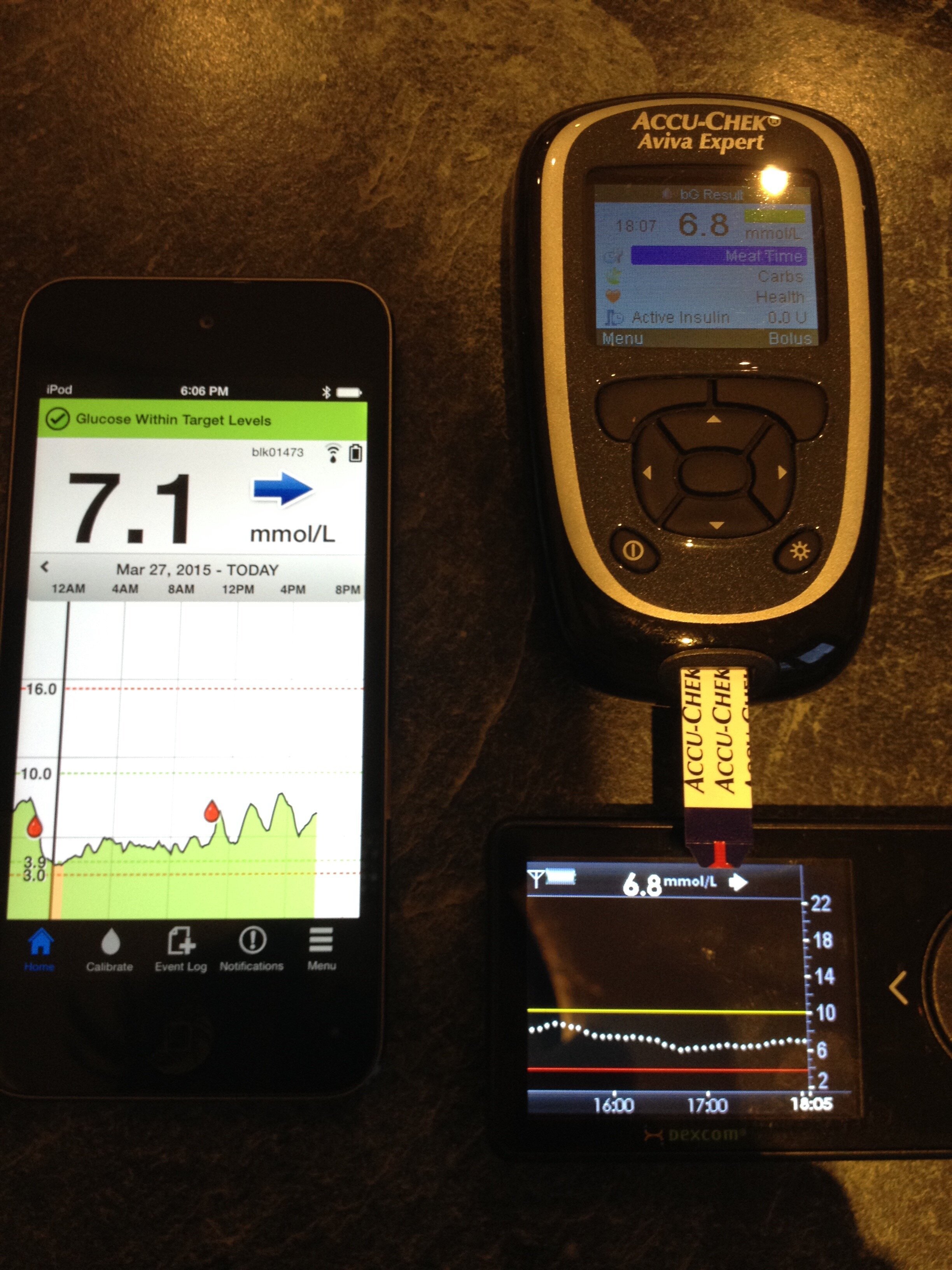
The Dexcom G4 sensors used a 7 day sensor to take interstitial fluid readings with a transmitter that lasted a year. Calibrations were done twice a day based on blood glucose readings. The Dexcom algorithm that converted readings to estimated blood glucose trends worked reasonably well for me, starting to give me confidence in using the alarms and historic trends for decision making.
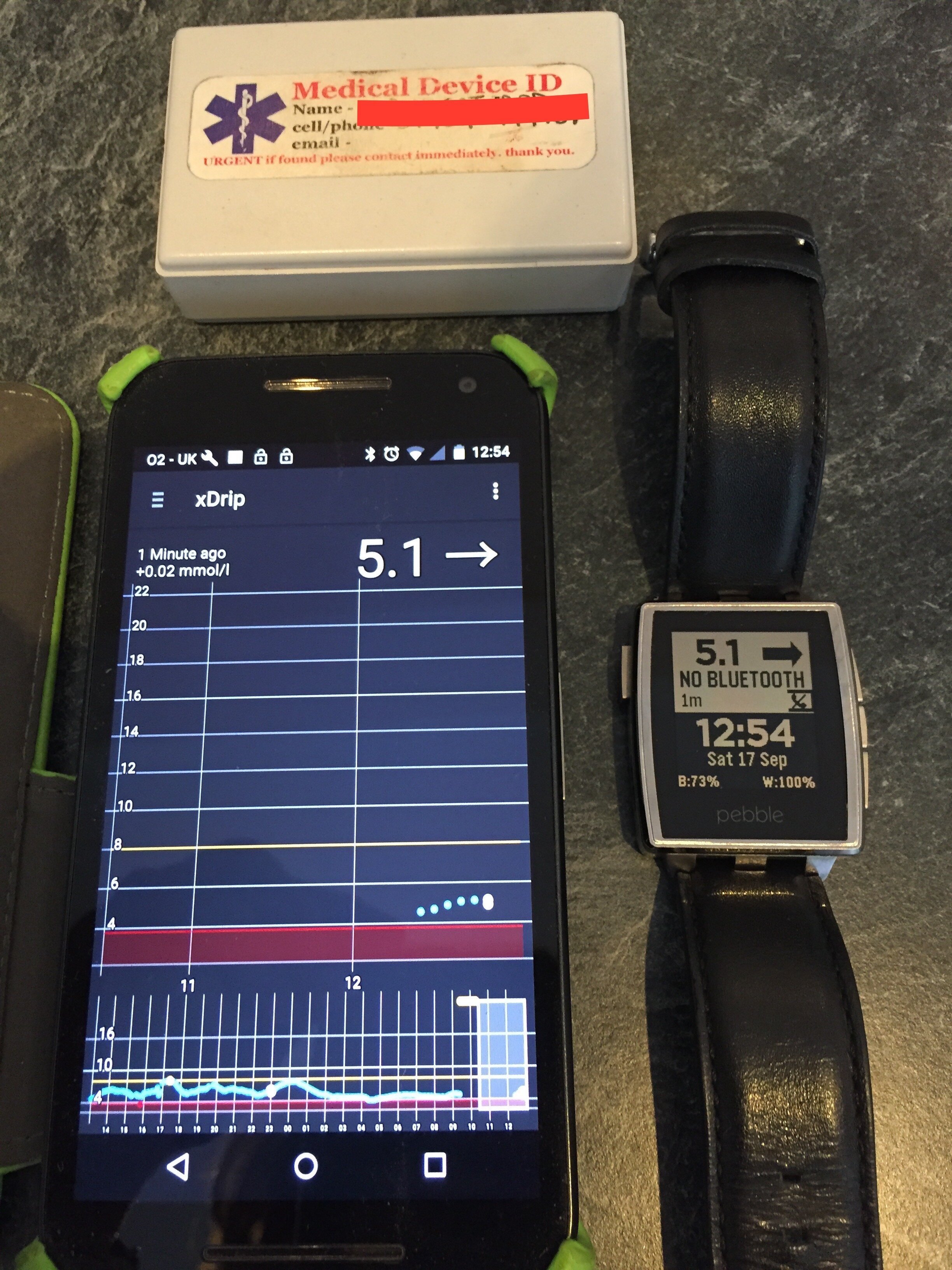
I moved to combining the Dexcom G4 with xDrip software developed by Stephen Black, a member of the DIY type 1 community. I attended a “build” party where I was shown how to solder the necessary devices to take transmitter readings from the G4 and send them to an android phone with xDrip software installed. Slightly more kit had to be carried around but sensors could be restarted which I found allowed me to extend their accurate usage to 30 instead of 7 days and I could link readings to a pebble watch, giving me easy access to data on my wrist!
Abbott developed the flash glucose monitoring system – Libre. I placed my first order in September 2016 to compare it to the Dexcom CGM. It was cheaper and sensors lasted 2 weeks but it was quite different from a CGM. The same interstitial fluid readings were used but rather than this data being read constantly with results pushed to a device that was capable of alarming for low or high blood glucose readings, trends could only be obtained by scanning the supplied rechargeable reader over the sensor. I found readings were significantly less accurate. When wearing both, the Libre would consistently read significantly lower suggesting that I should be treating low blood glucose levels when the CGM and blood glucose verification suggested no such action was needed. I also “liked” the reassurance of overnight alarms only available with the CGM.
Research into monitoring is ongoing. I have participated in two studies over the last six years looking at alternatives to existing CGM monitoring.
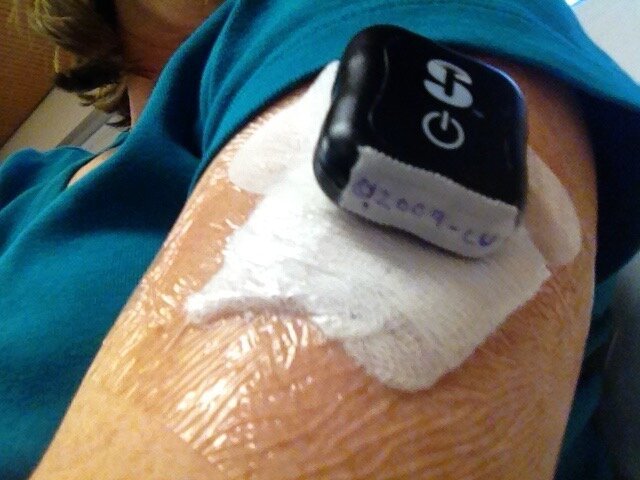
In March 2015, I took part in the Precise trial where an Eversense CGM was implanted into my upper arm. A rechargeable transmitter was worn over the top of the implanted sensor with estimated glucose readings sent to an ipod. Apart from needing to recharge the transmitter daily, which could easily be accomplished whilst in the shower, the implanted sensor lasted three months. The benefit from this freedom was immense. Rushed mornings when packing two youngsters out of the house for school and getting myself ready for work were made even more stressful when adding in set changes for the insulin pump or CGM sensor changes. With their all having different usage times, you cannot co-ordinate processes to choose one day of the week when you put aside time to administer all that needs to be done in managing type 1. To have three months “freedom” from CGM duties saved a considerable amount of administration time. Readings were accurate, but the sensor struggled to read in extremes of temperature. The other key downside was the need for a trained medical professional to remove a used sensor and insert a new one. I found that once I had become reliant on the data provided by CGMs, being without that support for any length of time, even if only several days, left me feeling very vulnerable.
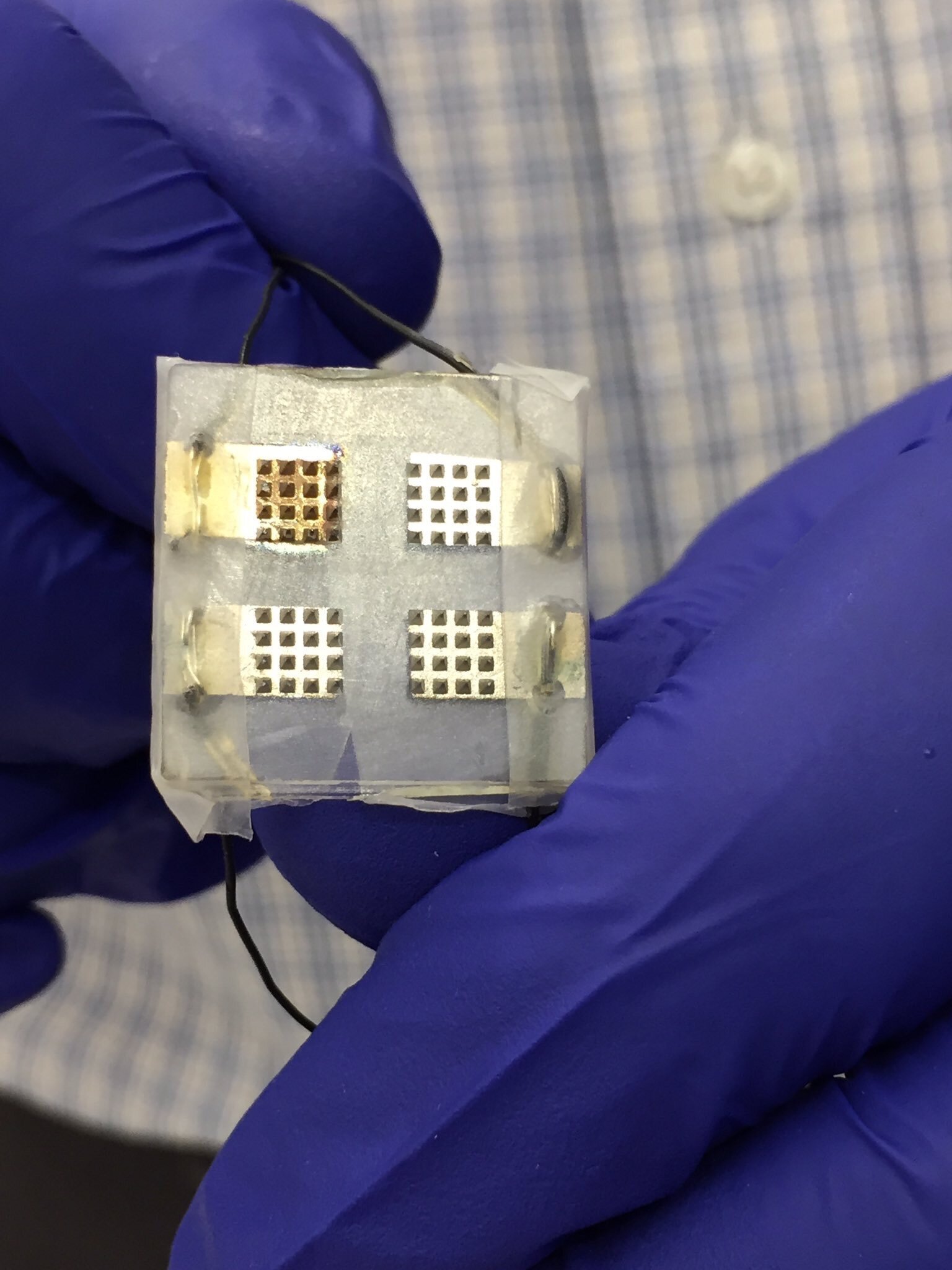
In September 2017, I participated in research undertaken by Imperial College London investigating the use of a microprobe CGM. It consisted of a small wearable patch about 1cm² worn on the forearm and containing small microprobes about 1mm in length. It was felt that development of this technology would offer certain advantages over existing devices and in using multiple microscopic sensors had the potential for improved accuracy. Only phase one of this research went ahead.
I am now self-funding my use of the latest version of Dexcom – the G6. Sensors now last for 14 days but transmitters need replacing every 3 months. The technology no longer needs twice daily calibration and is deemed accurate enough to use for dosing decisions without blood testing. I find it can still take 24 hours for the readings from a newly inserted sensor to “settle down” and I choose to perform at least 2 blood tests a day to give confidence about its continuing accuracy of measurement. CGMs are still not perfect, but I have identified the situations when they are less likely to be accurate for me and have adapted my behaviour accordingly.
My one frustration continues to be the pre-set manufacturer alarms. CGM results lag behind blood glucose results by anywhere from 5-15 minutes. I also find that if I have extended periods of inactivity, the CGM reading takes even longer to catch up. I have treated a low blood glucose entering a theatre auditorium, have undertaken a blood glucose check in the dark to know that I am no longer low, yet due to my lack of physical movement, my CGM readings stubbornly refuse to rise. There is a low alarm that is clearly audible and cannot be silenced unless you switch the device off. I resent the medtech company’s having this effect on my life and enjoyment of an evening out. I have chosen to self-fund this device; I use the data responsibly and if anything, an alarm in these circumstances will lead me to knowingly overtreat a low blood glucose because of my concern at spoiling other people’s enjoyment of a night out, when they do not understand the reasons for the alarm they hear. Even if it requires health care professional approval, I would like a patient to have the option of turning off the alarm.
We have come a long way in my nearly 50 years of monitoring, and development of the CGM, like the insulin pump, has been another game changer. Having the ability to see how my body responds to the many factors known to affect blood glucose levels now allows me the ability to step in and take remedial action, to limit the number of extreme high and low blood glucose events suffered. This in turn has allowed me to safely reduce my HbA1c further, which should help to keep my risk of suffering from the long-term complications associated with type 1 at a reduced level.
I finish by reproducing and modifying a tweet, originally posted by Tim Street, to help to explain the impact of the developments in these new technologies, to those living with type 1.
A urine test is like using a printed road atlas you have had in the car for several years, to find your way. It fails to recognise changed access or new roads built in the intervening years.
A blood glucose test is being shown your location - a point on a map. It is where you are now but offers no information about how you have got there and where you will be going next.
A CGM trend is like using a satnav. It shows you the route you have taken to get to where you are now, and how you are moving forwards.
A closed loop system where a CGM communicates with an insulin pump is like a satnav with rerouting capabilities. As with the CGM trend, it shows the route you have taken to get to where you are now but will make insulin changes for you, to help to keep you on the route you wish to follow.
I hope to gain access to a closed loop system in my 50th year of diagnosis.
Footnotes:
1. Ketones are released when there is a severe lack of insulin in the body. If left unchecked, ketones build up and make the blood become acidic leading to a state called diabetic ketoacidosis (DKA). If not detected and treated, DKA is life-threatening.